Effect of Chemical Reaction Rates on Aero-Heating Predictions of Re-entry Flows
The present work deals with the sensitivity of aero-heating predictions
to variations in chemical reaction rates in high enthalpy flows over
re-entry capsules. Within this scope, numerical simulations are performed by varying the reaction rate constants over their uncertainty range.
FIRE II re-entry capsule at 35 km altitude is chosen as the test case.
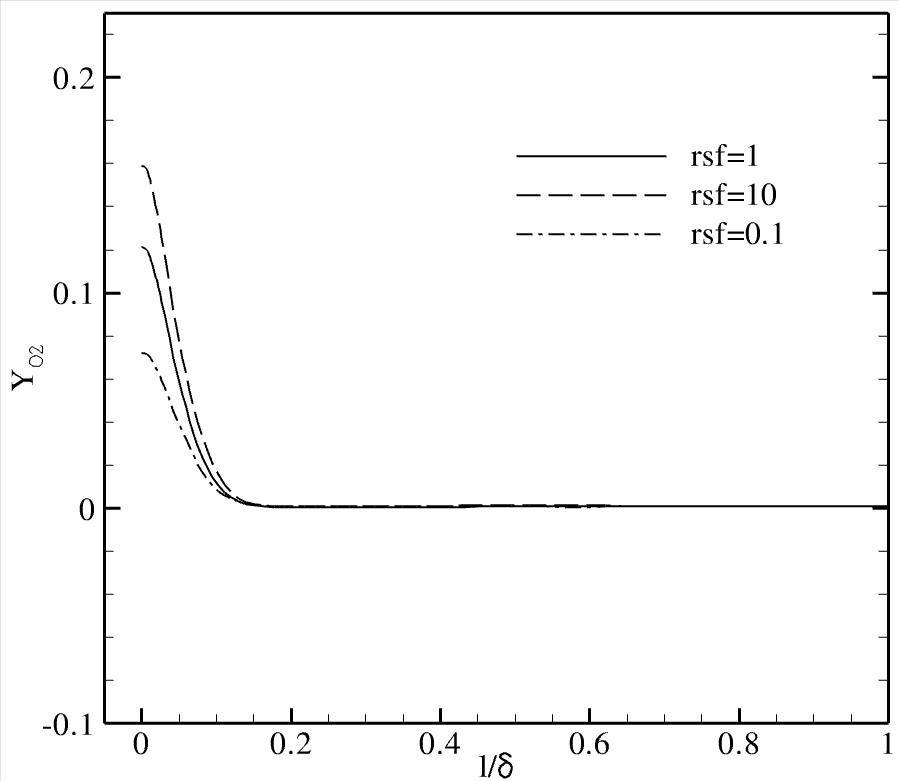
At these conditions, recombination of oxygen atoms is the dominant
chemical reaction in the thermal boundary layer near the wall.
The flow solutions computed by altering the reaction rates are carefully analyzed to understand the physical effects that
influence the surface heat transfer rate.
It is found that the chemical state of the gas i.e. equilibrium,
non-equilibrium or frozen, and the extent of recombination reactions in
the boundary layer are the critical factors that determine the
sensitivity of the heating rate to variations in the reaction rate
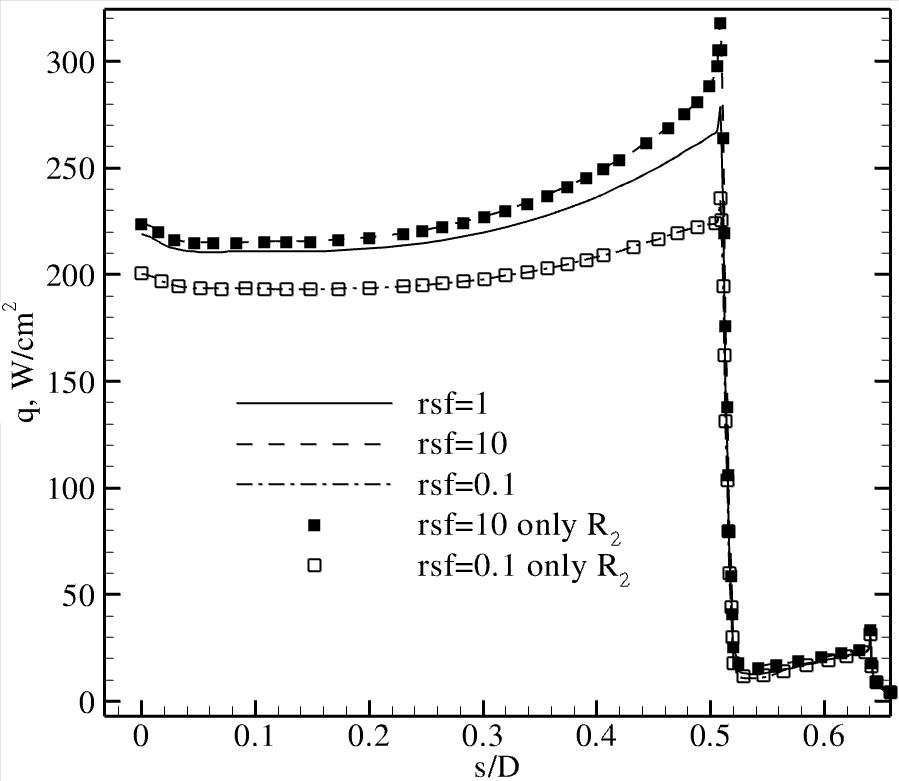
constants. The analysis is carried out at different points on the vehicle surface,
and the heating rate sensitivity is found to be highest at the
shoulder. This is due to the non-equilibrium chemical state of the gas
prevailing in the shoulder expansion region.
By comparison, the equilibrium state of the gas on the forebody and the
frozen chemistry on the afterbody result in lower sensitivity of the
heating rate to variations in the chemical reaction rates.
Ref: D.S.K Reddy and K. Sinha, Effect of chemical reaction rates on aero-heating predictions of re-entry flows, Accepted, Journal of Thermophysics and Heat Transfer.
.
Representative results: